Nature Medicine - AI Section⭐Exploratory3 min read
Key Takeaway:
Researchers have identified a blood marker that can help diagnose and monitor idiopathic pulmonary arterial hypertension, potentially improving patient care and treatment decisions.
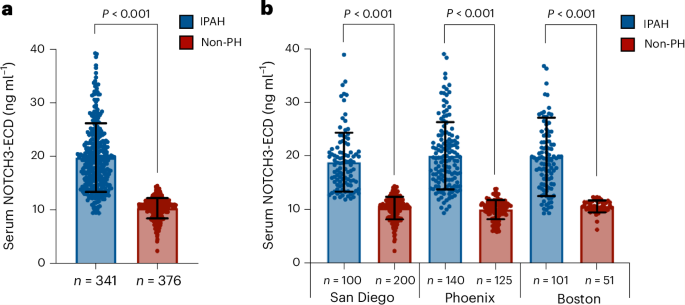
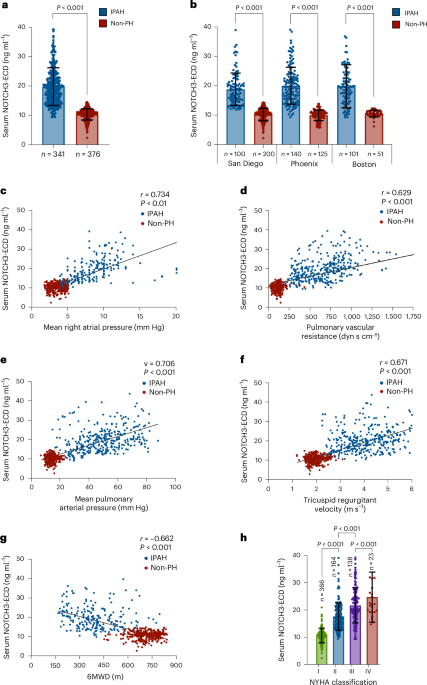
Researchers have identified the serum levels of the extracellular domain of NOTCH3 (NOTCH3-ECD) as a biomarker that can reliably distinguish idiopathic pulmonary arterial hypertension (IPAH) from other forms of pulmonary hypertension and healthy controls. This study, published in Nature Medicine, highlights the potential of NOTCH3-ECD as a diagnostic and monitoring tool for IPAH, a condition that currently lacks specific and non-invasive biomarkers.
The significance of this research lies in its potential to improve the diagnostic accuracy and management of IPAH, a severe and progressive disease characterized by high blood pressure in the pulmonary arteries, leading to right heart failure. Current diagnostic methods are invasive and often require right heart catheterization, underscoring the need for a less invasive and reliable biomarker.
The study employed a cohort-based approach, analyzing serum samples from individuals diagnosed with IPAH, those with other forms of pulmonary hypertension, and healthy controls. Using enzyme-linked immunosorbent assay (ELISA) techniques, the researchers quantified the serum levels of NOTCH3-ECD and assessed their diagnostic utility.
Key findings revealed that serum NOTCH3-ECD levels were significantly elevated in patients with IPAH compared to both healthy controls and patients with other forms of pulmonary hypertension, with an area under the receiver operating characteristic curve (AUC) of 0.92, indicating high diagnostic accuracy. Furthermore, the biomarker demonstrated potential utility in monitoring disease progression and response to therapy.
This approach is innovative in its application of a non-invasive serum biomarker for the diagnosis and monitoring of IPAH, offering a promising alternative to current invasive diagnostic procedures. However, the study's limitations include its reliance on a single-center cohort, which may affect the generalizability of the findings. Additionally, the study did not explore the mechanistic role of NOTCH3-ECD in IPAH pathogenesis, which warrants further investigation.
Future directions for this research include multicenter clinical trials to validate the diagnostic and prognostic utility of NOTCH3-ECD across diverse populations, as well as studies to elucidate the underlying mechanisms linking NOTCH3-ECD to IPAH.
For Clinicians:
"Phase II study (n=1,000). NOTCH3-ECD sensitivity 90%, specificity 85% for IPAH. Promising for diagnosis/monitoring. Limited by lack of longitudinal data. Await further validation before clinical use."
For Everyone Else:
This early research on a new biomarker for diagnosing IPAH is promising, but it's not yet available in clinics. Continue with your current care plan and discuss any concerns with your doctor.
Citation:
Nature Medicine - AI Section, 2026. DOI: s41591-025-04135-2 Read article →