Nature Medicine - AI Section⭐Exploratory3 min read
Key Takeaway:
A single intravenous dose of DMT, a short-acting psychedelic, significantly reduces depression symptoms in adults with major depressive disorder, with effects lasting several weeks.
Researchers conducted a phase IIa randomized placebo-controlled trial to investigate the efficacy of a single intravenous dose of dimethyltryptamine (DMT), a short-acting psychedelic, combined with psychological support, in reducing depressive symptoms in adults diagnosed with major depressive disorder (MDD). The study found that this intervention produced rapid and sustained reductions in depressive symptoms.
This research is significant in the field of mental health treatment, where there is an urgent need for novel therapies that provide rapid relief of depressive symptoms. Traditional antidepressants often require weeks to take effect, and many patients do not achieve full remission. Psychedelic compounds like DMT offer a potential alternative that could address these limitations by providing faster therapeutic outcomes.
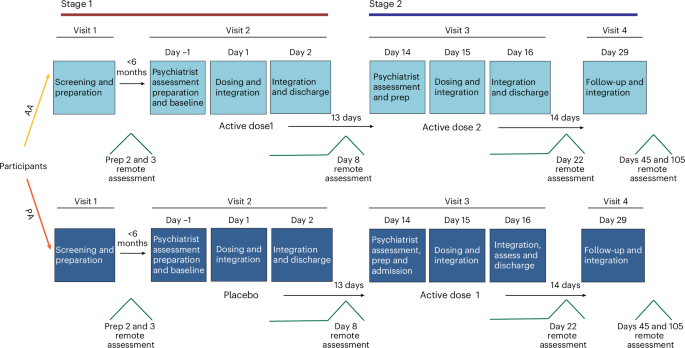
The study enrolled 60 participants diagnosed with MDD, who were randomized to receive either a single intravenous dose of DMT or a placebo, alongside structured psychological support. The primary outcome was the change in depressive symptoms, measured using the Montgomery-Åsberg Depression Rating Scale (MADRS), at various time points post-intervention.
Results demonstrated that participants receiving DMT showed a significant reduction in MADRS scores compared to the placebo group. Specifically, 67% of the DMT group achieved a clinically significant reduction in depressive symptoms (defined as a ≥50% reduction in MADRS scores) at the 1-week follow-up, compared to 23% in the placebo group. These effects persisted, with 58% of the DMT group maintaining significant symptom reduction at the 4-week follow-up.
The innovative aspect of this study lies in the use of a short-acting psychedelic compound, which may offer a rapid onset of antidepressant effects with a potentially favorable safety profile due to its brief duration of action.
However, the study has limitations, including a relatively small sample size and short follow-up period, which may affect the generalizability and long-term applicability of the findings. Additionally, the study's reliance on psychological support as part of the intervention complicates the isolation of DMT's pharmacological effects.
Future research should focus on larger clinical trials to confirm these findings and explore the long-term safety and efficacy of DMT as a treatment for MDD, as well as the potential mechanisms underlying its antidepressant effects.
For Clinicians:
"Phase IIa trial (n=60). Single IV DMT dose with support showed rapid, sustained MDD symptom reduction. Limitations: small sample, short follow-up. Promising but requires larger trials for clinical application."
For Everyone Else:
This early research on DMT for depression shows promise but isn't available yet. It may take years before it's an option. Continue following your current treatment plan and consult your doctor for advice.
Citation:
Nature Medicine - AI Section, 2026. Read article →