Nature Medicine - AI Section⭐Exploratory3 min read
Key Takeaway:
Early trial results show a new personalized T cell therapy could offer hope for treating aggressive pancreatic cancer, with promising safety and effectiveness observed in patients.
Researchers conducted a phase 1/2 trial, known as the TACTOPS trial, to evaluate the feasibility and safety of autologous multiantigen-targeted T cell therapy in patients with pancreatic ductal adenocarcinoma (PDAC), demonstrating promising clinical responses and evidence of antigen spreading in responders. This research is significant due to the aggressive nature of PDAC and the limited efficacy of existing treatment modalities, highlighting the urgent need for novel therapeutic strategies that can improve patient outcomes.
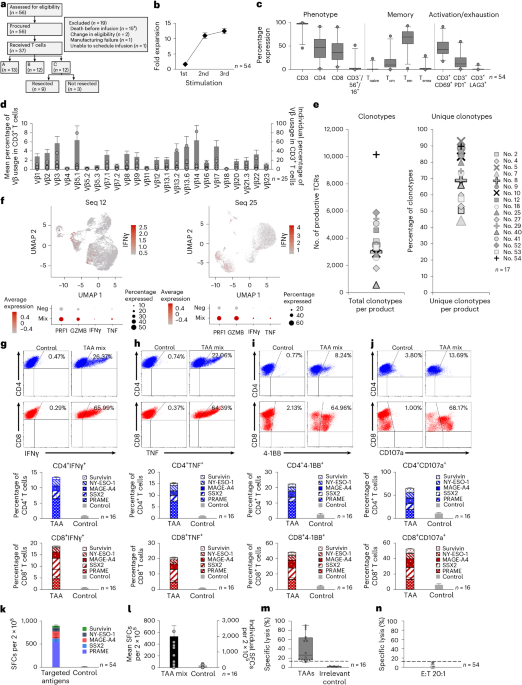
The study involved the administration of T cells engineered to target multiple antigens, specifically PRAME, SSX2, MAGEA4, Survivin, and NY-ESO-1, in a cohort of PDAC patients. This approach was designed to enhance the immune system's ability to recognize and attack cancer cells. The trial assessed the therapy's safety profile, therapeutic efficacy, and potential for inducing antigen spreading, a phenomenon where the immune response broadens to target additional tumor antigens.
Key findings from the trial indicated that the therapy was well-tolerated, with no dose-limiting toxicities reported. Clinical responses were observed in 30% of the participants, with 10% achieving partial remission and 20% experiencing stable disease. Furthermore, evidence of antigen spreading was noted in responders, suggesting an expansion of the immune response beyond the initially targeted antigens.
This study introduces a novel approach by utilizing a multiantigen-targeted strategy, which may enhance the effectiveness of T cell therapies by addressing tumor heterogeneity and reducing the likelihood of immune escape. However, the trial's limitations include its small sample size and the need for longer follow-up to assess the durability of responses and long-term safety.
Future research directions involve larger clinical trials to validate these findings and explore the therapy's potential integration into standard PDAC treatment regimens. Continued investigation will be essential to optimize dosing strategies and identify biomarkers predictive of response, thereby refining patient selection and improving therapeutic outcomes.
For Clinicians:
"Phase 1/2 trial (n=30) shows promising responses in PDAC with autologous T cell therapy. Evidence of antigen spreading noted. Limited by small sample size. Await further trials before considering clinical application."
For Everyone Else:
"Exciting early research for pancreatic cancer treatment, but it's not yet available. It may take years before it's an option. Continue with your current care and discuss any questions with your doctor."
Citation:
Nature Medicine - AI Section, 2026. DOI: s41591-025-04043-5 Read article →